The Clinical Trials Unit
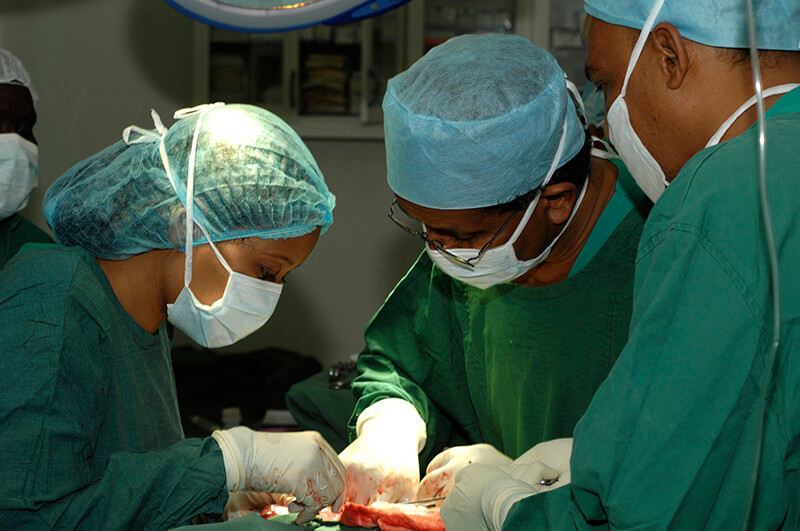
The Clinical Trials Unit at the Mycetoma Research Center was established with the vision of advancing innovative research and significantly improving health outcomes for individuals affected by mycetoma, a neglected tropical disease that poses substantial health, economic, and societal challenges. This specialised unit is designed to serve as a critical platform for conducting rigorous clinical research aimed at discovering new treatments, enhancing diagnostic accuracy, and informing public health policies to better control and prevent the disease.
Its core objectives include the development of at least one novel anti-mycetoma drug within a five-year timeframe, the implementation of high-precision AI-based diagnostic tools capable of rapidly and accurately identifying mycetoma infections, and the creation of comprehensive disease maps and risk profiles that can be used by public health authorities to allocate resources effectively and design targeted intervention strategies.
Beyond these immediate research goals, the unit is committed to building and strengthening local research capacity by providing training and mentorship to researchers and clinicians in cutting-edge methodologies, including clinical trial design, data management, biostatistics, and ethical conduct of research. This capacity-building component aims to ensure sustainability and foster a new generation of experts equipped to continue advancing mycetoma research.

Additionally, the unit actively seeks to secure sustained funding through national and international research grants, collaborations, and partnerships with academic institutions, governmental agencies, and non-governmental organisations. These collaborations are vital for expanding research scope, sharing expertise, and facilitating the translation of research findings into practical health solutions.
The overarching goal of the Clinical Trials Unit is to reduce the burden of mycetoma by decreasing morbidity and mortality rates through the development of effective therapies and diagnostics. Economically, the unit aims to lower healthcare costs associated with treating advanced cases and complications of mycetoma, while also improving the productivity and quality of life of affected populations. Scientifically, the unit aspires to position the Mycetoma Research Center as a leading global authority in tropical disease research, contributing valuable knowledge to the scientific community. From a societal perspective, the unit’s efforts are geared toward increasing awareness, promoting early detection, and implementing preventive measures, all of which contribute to fostering healthier and more resilient communities.

The activities of the Clinical Trials Unit encompass all phases of clinical research, starting with designing detailed trial protocols for testing new drugs and diagnostic methods and obtaining the necessary ethical and regulatory approvals to ensure compliance with international standards. Participant recruitment is carried out carefully, with eligible patients enrolled based on strict inclusion and exclusion criteria, followed by baseline assessments and continuous monitoring throughout the trial period. The implementation of the trials adheres strictly to the different Good Clinical, Laboratory and Financial Practice (GCP) standards to ensure the safety, rights, and well-being of participants. Data collection is conducted systematically, utilising electronic data capture systems to enhance accuracy, security, and ease of analysis. The collected data undergo rigorous analysis to evaluate safety profiles, efficacy of interventions, and diagnostic accuracy, with results compiled into comprehensive interim and final reports for stakeholders, regulatory bodies, and scientific dissemination.
Safety monitoring is a fundamental aspect of the unit’s activities. Data Safety Monitoring Boards (DSMBs) are established to oversee participant safety throughout the trial, promptly recording and managing any adverse events to ensure the ethical conduct and protection of participants. Once trials are completed, the unit prioritises the dissemination of findings through publication in reputable scientific journals and presentations at conferences, facilitating knowledge sharing within the scientific community and among health policymakers. Additionally, collaboration with regulatory agencies is pursued to facilitate the approval and wider adoption of successful interventions. Post-trial follow-up is also a crucial component, focusing on monitoring the long-term safety and effectiveness of new therapies and diagnostics, as well as planning strategies for scaling up successful interventions to benefit broader populations.
The expected outcomes and impacts of the Clinical Trials Unit are significant. These include the successful development of at least one innovative anti-mycetoma drug within five years, the deployment of AI diagnostic tools with high accuracy, and the generation of detailed disease maps and risk profiles to guide tailored public health initiatives. The unit also aims to strengthen local research capacity through ongoing training programmes and to foster robust partnerships at both national and international levels, thereby supporting sustained research efforts. The health impact of these initiatives is expected to be profound, leading to reduced morbidity and mortality rates associated with mycetoma, while the economic benefits include decreased healthcare costs and improved productivity among affected populations. Scientifically, the unit’s work will position the Mycetoma Research Center as a global leader in tropical disease research, contributing to the global knowledge base and fostering innovative approaches to disease management. Societally, the increased awareness and preventive strategies promoted by the unit are anticipated to result in healthier communities with fewer cases and less severe disease progression.
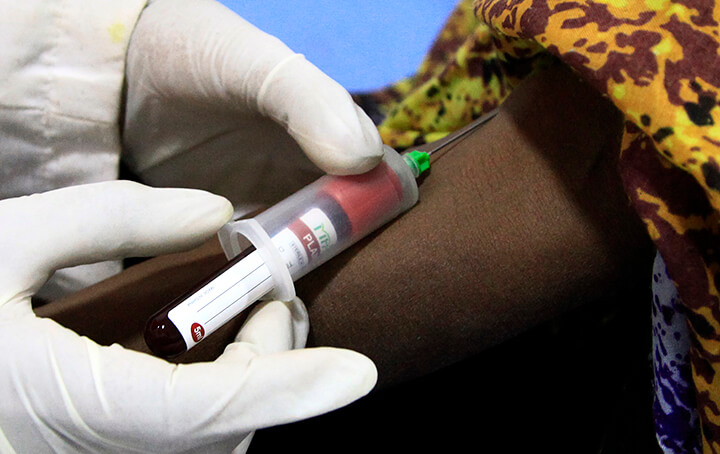
The Clinical Trials Unit at the Mycetoma Research Centre is a comprehensive and strategic initiative that combines cutting-edge research, capacity building, and collaborative efforts to address the pressing challenges posed by mycetoma. Its activities and outcomes are designed not only to advance scientific knowledge but also to translate research into tangible health benefits, ultimately contributing to the reduction of disease burden and the promotion of healthier, more resilient societies worldwide.
Activities
Protocol Development and Ethical Approval
Design clinical trial protocols for testing new drugs and diagnostic methods.
Obtain necessary ethical and regulatory approvals.
Participants’ Recruitment and Monitoring
Enrol eligible patients according to the specified inclusion and exclusion criteria.
Conduct baseline assessments and monitor participants throughout the trial.
Implementation of Trials
Administer investigational therapies or diagnostics according to trial protocols.
Ensure adherence to Good Clinical Practice (GCP) standards.
Data Collection and Management
Collect clinical, laboratory, and imaging data systematically.
Use electronic data capture systems for accuracy and security.
Data Analysis and Reporting
Analyse trial data to evaluate safety, efficacy, and diagnostic accuracy.
Prepare interim and final reports for stakeholders and regulatory bodies.
Safety Monitoring
Establish Data Safety Monitoring Boards (DSMB) to oversee participant safety.
Record and manage adverse events.
Dissemination of Findings
Publish results in scientific journals and present at conferences.
Collaborate with regulatory agencies to facilitate the potential approval of successful interventions.
Post-Trial Follow-up
Monitor long-term safety and effectiveness of therapies and diagnostics.
Plan for scaling up successful interventions for wider use.
See also: El Hassan Centre
